Research Highlight
Accumulated Excess Protein Linked To Development Of Parkinson’s Disease By Progressively Disrupting Neuronal Function And Viability
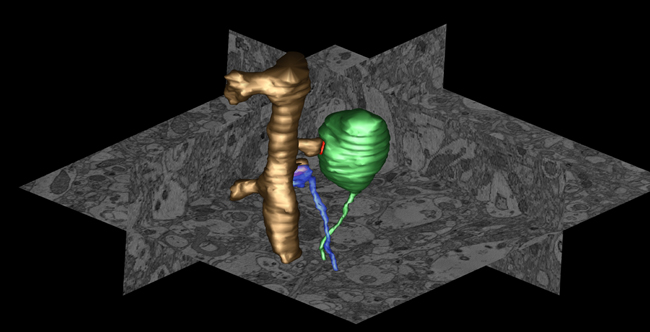
Figure Caption: Example of an atypical presynaptic terminal in neocortex of alpha-synuclein (AS) transgenic mice. Using serial block face scanning EM we observed in neocortex of AS transgenic mice enlarged nerve terminals massively filled with an endomembrane network. 3D volume segmentation shows the enlarged terminal (green) forming a synapse (postsynaptic density is in red) with a dendritic spine (golden) compared to a normal terminal (blue) forming a synapse with an adjacent spine on the same dendrite.
January, 2013 La Jolla -- NCMIR scientists have shown that overexpression of a protein called alpha-synuclein appears to disrupt vital recycling processes in neurons, starting with the terminal extensions of neurons and working its way back to the cells’ center, with the potential consequence of progressive degeneration and eventual cell death. Published in the February 6, 2013 issue of The Journal of Neuroscience, this study has major implications for more fully understanding the causes and mechanisms of Parkinson’s disease (PD), a neurodegenerative movement disorder that affects an estimated 1 million Americans. Parkinson’s disease is characterized by the gradual destruction of select brain cells that produce dopamine, a neurotransmitter involved in regulating movement and emotion. Symptoms include increasing loss of muscle and movement control. While most cases are sporadic, there are also inherited forms of PD linked to specific gene mutations and modifications.
The NCMIR team headed by Daniela Boassa and Mark Ellisman together with Dr. Eliezer Masliah at UCSD and Mary Ann Yang and Julia M. George at the University of Illinois, Urbana, focused on alpha-synuclein. Using a variety of leading-edge imaging technologies, including a new fluorescent tagging technique developed for electron microscopy by UCSD Nobel laureate Roger Tsien’s lab and colleagues at NCMIR, the scientists investigated, at high resolution the effects of human wild-type alpha-synuclein overexpression both in vitro and in vivo recapitulating the clinical circumstances of increased expression of alpha-synuclein associated with the pathogenesis of sporadic as well as familial PD. They found that excess levels of alpha-synuclein accumulated in membrane systems of the presynaptic part of the synapse, the place where axons and dendrites of brain cells meet to exchange chemical signals for impulse propagation. As alpha-synuclein accumulates in the terminals, it causes profound perturbations of the membrane architecture leading to enlarged presynaptic terminals filled with membrane-bounded organelles such as tubulo-vesicular structures. Such structural abnormalities correlate with synaptic dysfunction and appears to hinder normal degradation and recycling processes in neurons. This would progressively impair the release of neurotransmitters. In time, the neurons might simply stop functioning and die. While other studies have noted that PD is characterized by progressive loss of vesicle traffic, and neurotransmitter release, this study provides for the first time a structural and mechanistic explanation for why that happens.
Funding for this research came, in part, from the NIH National Center for Research Resources (5 P41RR004050-24), the National Institute of General Medical Sciences (8 P41 GM103412-24), National Institutes of Health grants R01 GM086197-05, AG184440 and AG022074, as well as support from the Branfman Family Foundation and the Institute for Systems Biology, as part of the activities of a consortium of researchers linked to the Luxembourg Center for Systems Biomedicine’s research program on neurodegenerative disease.
Citation: Boassa D, Berlanga ML, Yang MA, Terada M, Hu J, Bushong EA, Hwang M, Masliah E, George JM, Ellisman MH (2013). Mapping the Subcellular Distribution of α-Synuclein in Neurons using Genetically Encoded Probes for Correlated Light and Electron Microscopy: Implications for Parkinson's Disease Pathogenesis. J Neurosci. 2013 Feb 6;33(6):2605-15. PMID: 23392688.Cell Metab 15:848-860.
Online version of the article in Journal of Neuroscience